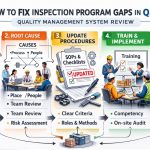
Introduction to Essential QMSR Compliance Tools
The medical device industry is witnessing a major regulatory shift. The FDA is moving from the old Quality System Regulation (QSR) to the new Quality Management System Regulation (QMSR). This change harmonizes US standards with the international ISO 13485:2016 framework. Consequently, companies must now find the right QMSR compliance tools to manage this transition. These tools are no longer optional but necessary for survival in the 2026 regulatory landscape.
For manufacturers, the goal is clear: maintain high-quality standards while satisfying federal investigators. Modern tools help achieve this by integrating risk management directly into the production process. They allow firms to move away from manual spreadsheets toward automated, real-time data capturing. Furthermore, the right software ensures that every design change and corrective action is fully traceable. This level of transparency is exactly what the FDA seeks during a modern inspection.
In this guide, we evaluate the top tools designed for the modern era. We explore how digitalization simplifies the FDA QMSR Inspection Program Rule Interpretation for your quality team. Whether you are a small startup or a large global firm, these tools will help you maintain an “always-ready” audit state. By choosing the correct technology, you can turn compliance from a hurdle into a competitive advantage.
Digitalization: The Backbone of QMSR Readiness
Modern compliance depends heavily on digitalization. The FDA now prioritizes data integrity and software validation during its visits. Therefore, your QMSR compliance tools must support the ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, and Accurate). Automated systems capture data at the source, which eliminates the risk of human error found in paper logs.
Investigators in 2026 frequently examine digital audit trails. They want to see exactly who made a change and why. Consequently, software that offers unalterable time-stamped records is vital. This technology supports your Medical Device QMSR Inspection Program Template by providing the evidence needed for every regulatory claim. Without these digital proofs, your facility faces a much higher risk of receiving a Form 483.
Furthermore, these tools allow for better remote collaboration. Quality teams can review documents and sign off on changes from anywhere. This agility is crucial for managing global supply chains. When you implement these digital solutions, you are not just buying software; you are building a resilient quality culture.
Top Tool Categories for Medical Device Quality Systems
To stay compliant, you need a suite of specialized tools. Each category addresses a specific part of the QMSR compliance tools requirement.
1. Automated Quality Management Systems (eQMS)
An eQMS is the central nervous system of your quality department. It manages everything from Document Control to CAPA. These systems ensure that you follow the Step-by-Step Medical Device Inspection Program for QMSR Compliance without missing a single milestone.
2. Risk Management Software
QMSR places risk at the center of the QMS. Dedicated risk management tools help you map potential hazards throughout the product lifecycle. They link your FMEA (Failure Mode and Effects Analysis) directly to your design controls.
3. Training Management Portals
Training is a frequent target for FDA investigators. You must prove that every employee is competent in their specific role. Automated portals track training completion and store certificates in an easily searchable database.
Using Software to Bridge the Gap: QSR to QMSR
Many firms struggle with the transition because they rely on outdated methods. However, the right QMSR compliance tools act as a bridge. They help you map your existing 21 CFR 820 processes to the new ISO-aligned requirements. This automated mapping prevents the gaps that often lead to regulatory findings.
When you learn how to implement medical device inspection program QMSR, you realize that visibility is everything. Software provides “dashboards” that alert you to overdue tasks or pending investigations. This proactive approach keeps your QMS healthy and avoids the “panic-mode” audits of the past.
Moreover, these tools simplify the generation of summary reports. Instead of spending weeks preparing for an auditor, you can generate a compliance report with a few clicks. This efficiency allows your quality team to focus on actual product improvement rather than just paperwork.
Mock Audits and the Role of Verification Technology
Before a real FDA investigator arrives, you must test your systems. This is where mock audits become essential. Specialized software can simulate the FDA’s investigative path. It guides your team through a “stress test” of your “front room” and “back room” logistics.
If you are unsure if your facility is prepared, ask yourself: Do You Need a Mock FDA Audit? Benefits and What to Expect. The best tools for this process provide scoring rubrics. These rubrics help you quantify your audit readiness. They highlight exactly where your documentation is weak or where your staff needs more training.
A successful mock audit provides the confidence needed for the real event. It ensures that your QMSR Inspection Program Checklist for Medical Devices is actually working. Technology allows you to track the resolution of any “mock findings” so you are 100% ready when the federal badge appears.
Data Integrity and 21 CFR Part 11 Compliance
As manufacturing becomes more automated, the importance of Part 11 increases. Your QMSR compliance tools must be fully validated for their intended use. This means you must have documented proof that the software functions as described by the vendor.
FDA investigators will check your validation protocols. They want to see that you have tested for both “intended” and “unintended” system behaviors. Modern tools often come with “validation starter kits.” These kits provide templates for IQ/OQ/PQ (Installation, Operational, and Performance Qualification), saving your team hundreds of hours.
Remember, a system is only as good as the data it contains. You must establish strict access controls to prevent unauthorized data entry. Use tools that support multi-factor authentication (MFA) and strong encryption to protect your sensitive design and manufacturing data.
Conclusion: Choosing the Right QMSR Toolset
Selecting the right QMSR compliance tools is a strategic decision for the future of your company. In 2026, the FDA expects manufacturers to use modern technology to manage their quality systems. By moving away from manual, paper-based processes, you significantly reduce your regulatory risk. You also gain deeper insights into your manufacturing efficiency.
Compliance should not be seen as a burden, but as a commitment to patient safety. The tools you choose reflect that commitment. Invest in technology that offers scalability, data integrity, and user-friendly interfaces. By doing so, you ensure your innovations reach the market faster and stay there longer. Start your digital transformation today to secure your place in the global medical device market.
Frequently Asked Questions (FAQs)
1. Can I use general project management software for QMSR? Generally, no. You need software specifically designed for 21 CFR Part 11 compliance to ensure the necessary data integrity and audit trails required by the FDA.
2. How long does it take to implement a new eQMS? A full implementation typically takes 3 to 9 months, depending on the size of your organization and the complexity of your legacy data migration.
3. Will the FDA accept electronic signatures during an inspection? Yes, the FDA accepts electronic signatures as long as they comply with the requirements of 21 CFR Part 11.
4. What is the most important feature of a QMSR tool? The audit trail is arguably the most important feature, as it provides the ultimate proof of compliance for every action taken within the system.
5. How does software help with ISO 13485:2016 alignment? Software helps by automatically structuring your QMS to match the clauses of the ISO standard, ensuring that every required element is addressed.
6. Do small companies really need automated compliance tools? Yes. In 2026, the regulatory burden is the same regardless of company size. Automation allows small teams to manage complex compliance tasks efficiently.
References and Technical Citations
FDA QMSR Final Rule Official Documentation: https://www.fda.gov/medical-devices/quality-system-regulation-amendments This official document details the legal shift to QMSR and the mandatory harmonization with ISO 13485:2016 for all medical device manufacturers.
ISO 13485:2016 Standard for Medical Devices: https://www.iso.org/standard/59752.html The primary international standard that defines the requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices.
FDA Inspection Guides (QSIT) Technical Manual: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/quality-system-inspection-technique-qsit A detailed guide used by federal investigators to evaluate the four major subsystems of a medical device quality management system.
FDA Guidance on Data Integrity and CGMP: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-compliance-cgmp-guidance-industry Provides technical expectations for manufacturers regarding the accuracy, reliability, and security of both paper and electronic records.
IMDRF Software as a Medical Device (SaMD) Framework: https://www.imdrf.org/documents/software-as-a-medical-device-samd-key-definitions-and-framework The international framework for defining and validating software used in a medical context, supporting global regulatory harmonization.
European Medical Device Regulation (EU MDR 2017/745): https://health.ec.europa.eu/medical-devices-sector/new-regulations_en The EU’s comprehensive regulation for medical devices, which works alongside the FDA’s QMSR to drive international quality management standards.