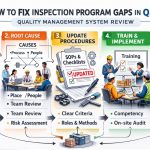
Introduction to QMSR Medical Device Regulation Inspection Guidance
The regulatory environment for medical technology is currently undergoing a transformative shift. Recently, the FDA finalized the Quality Management System Regulation (QMSR), which replaces the long-standing Quality System Regulation (QSR). This move represents a strategic effort to align US federal requirements with international quality standards. Consequently, understanding the QMSR Medical Device Regulation Inspection Guidance is now a fundamental requirement for all manufacturers operating in the US market. This guidance serves as the primary roadmap for how investigators will evaluate your facility in 2026.
For manufacturers, this harmonization with ISO 13485:2016 simplifies global compliance but introduces new technical expectations. The FDA now prioritizes the effectiveness of your Quality Management System (QMS) over static documentation. Therefore, staying informed about these changes is vital for maintaining market access and protecting patient safety. The new inspectional approach focuses on how risk management permeates every layer of your organization. This shift requires a departure from traditional “checkbox” auditing toward a more integrated, process-oriented evaluation.
In this comprehensive guide, we analyze the core pillars of the new guidance document. We explore the tactical shifts required for audit readiness and the impact of digital integrity on compliance outcomes. Whether you are transitioning an existing system or building one from scratch, these insights will help you navigate the modern FDA landscape with confidence. By adopting a proactive stance, you can ensure your innovative medical devices reach the market without the delay of regulatory intervention.
The Strategic Shift: Harmonizing with ISO 13485
The primary objective of the QMSR is to reduce the regulatory burden by converging domestic and international requirements. Although the fundamental goals of quality remain unchanged, the methodology for proving compliance has evolved. Specifically, the QMSR Medical Device Regulation Inspection Guidance emphasizes the integration of ISO 13485:2016 standards. This means that investigators will now look for high-level alignment with international best practices during every site visit.
One major change involves the explicit requirement for risk-based thinking throughout the QMS. Previously, many firms treated risk management as a standalone activity confined to the design phase. Under QMSR, however, you must demonstrate how risk assessments influence purchasing, production, and even post-market surveillance. Investigators will seek objective evidence that data-driven risk analysis informs your most critical quality decisions.
To manage this transition effectively, you must understand the underlying legal changes. A detailed FDA QMSR Inspection Program Rule Interpretation can provide the necessary context for your quality team. Mapping your current 21 CFR 820 procedures to the new QMSR framework is the first step in identifying systemic gaps that could lead to a Form 483.
Core Pillars of the QMSR Inspection Framework
The new guidance document structures inspections around several fundamental pillars. Each pillar must be robust enough to withstand the scrutiny of a federal investigator.
Management Responsibility and Oversight
The FDA expects senior leadership to be the primary drivers of quality. In a QMSR inspection, investigators will evaluate how management monitors the effectiveness of the QMS. This includes reviewing management review minutes and ensuring that adequate resources are allocated to maintain compliance. Management must prove that quality is a core business strategy, not just a departmental function.
Integrated Risk Management
Risk management is the “red thread” that connects every subsystem in the QMSR. It begins at the concept phase and continues throughout the product’s life. During an audit, the investigator will trace a failure mode from the risk management file to the manufacturing floor. They want to see that the controls you designed are actually functioning in a real-world production environment.
Design and Development Controls
While the QMSR maintains high standards for design controls, it adds a specific focus on design transfer and validation. This requires a clear, documented link between design outputs and final manufacturing specifications. For firms approaching a major product launch, knowing how to satisfy the Medical Device Inspection Program QMSR Requirement is essential for a successful submission.
Operational Excellence in Audit Readiness
Maintaining a state of “always-ready” compliance is the gold standard for medical device firms. To achieve this, organizations must move away from the “panic-prep” model of the past. Instead, they should utilize a Medical Device QMSR Inspection Program Template to standardize their internal review processes. This ensures that every subsystem—from CAPA to Document Control—is evaluated consistently throughout the year.
Internal audits should be conducted with the same rigor as an actual FDA inspection. This includes conducting mock interviews with subject matter experts (SMEs) to ensure they can explain their processes clearly. If an SME cannot articulate how they manage risk in their specific area, it signals a lack of training to the investigator. Therefore, continuous training and competency assessments are vital components of your readiness strategy.
Furthermore, the “Back Room” logistics must be flawless. Investigators evaluate a company’s organization based on how quickly they can retrieve a requested record. If it takes your team more than 15 minutes to find a validation report, it suggests a lack of control over your documentation. Efficiency in the back room reflects a mature and organized quality culture in the front room.
Choosing Between Outsourced and Internal Audit Models
As companies adapt to the new guidance, a common strategic question arises: should the inspection program be managed internally or by external experts? This decision significantly impacts your long-term compliance posture. There are distinct Outsourced vs Internal QMSR Inspection Program Pros and Cons that management must weigh carefully.
Internal programs foster deep institutional knowledge and a stronger quality culture. However, they are susceptible to “tunnel vision,” where team members overlook habitual non-compliances. Conversely, outsourced audits provide an objective, third-party perspective and access to specialized regulatory experts who see trends across the entire industry. Many successful firms use a hybrid model, keeping daily quality tasks internal while hiring external consultants for high-stakes periodic audits.
Regardless of the model, you must ensure your auditors are trained on the latest QMSR interpretations. An auditor who still relies on old QSR checklists will miss the critical risk management and ISO-alignment gaps that FDA investigators now target. Consistency between your chosen model and your regulatory goals is the key to minimizing 483 risks.
The Strategic Role of Mock FDA Audits
A simulated inspection is arguably the most effective way to verify the health of your QMS. A mock audit serves as a high-pressure rehearsal that identifies vulnerabilities before they are discovered by the government. This process is invaluable for training your staff on how to interact with federal investigators with professionalism and accuracy.
A successful mock audit should be performed by objective experts who have no stake in the daily operations of the facility. These auditors will “stress test” your systems, looking for gaps in your QMSR Inspection Program Checklist for Medical Devices. They will evaluate your staff’s ability to defend their quality decisions under questioning.
If you are unsure whether your facility is ready for this investment, it is helpful to explore Do You Need a Mock FDA Audit? Benefits and What to Expect. A mock audit provides the objective evidence needed to prove that your integrated QMS is actually functioning as a unified whole. It transforms compliance from a theoretical goal into an operational reality.
Navigating CAPA and Post-Market Surveillance
Corrective and Preventive Action (CAPA) remains a primary focus area during any FDA visit. The QMSR Medical Device Regulation Inspection Guidance demands a more holistic approach to CAPA that addresses systemic root causes rather than just individual errors. Investigators will look for “stagnant” CAPAs that show no evidence of timely completion or effectiveness checks.
Your CAPA system must be directly linked to your post-market surveillance (PMS) data. When a complaint is received from the field, it should trigger a review of the existing risk assessment. If the failure mode was not previously identified, or if it is occurring more frequently than predicted, you must take corrective action. This “closed-loop” system is the hallmark of a high-functioning quality management system.
Failure to properly link these systems is a significant red flag. Investigators want to see that if a problem is identified in the hands of a patient, the manufacturer takes immediate and effective steps to prevent it from happening again. Transparency in your investigation process is the best way to avoid a Form 483 during this critical part of the inspection.
Conclusion: Embracing the Future of Quality Regulation
The transition to the QMSR Medical Device Regulation Inspection Guidance represents a positive step toward a more efficient and globally aligned regulatory environment. While the shift requires significant effort, it offers a clear roadmap for improving device quality and patient outcomes. By prioritizing risk-based thinking, embracing digital integrity, and utilizing tools like mock audits, manufacturers can turn compliance into a strategic asset.
Compliance in 2026 is not a destination but a continuous journey of improvement. As the FDA refines its oversight, the most successful companies will be those that integrate quality into the very fabric of their organization. Stay informed, stay proactive, and maintain a culture where every employee is a guardian of quality. By doing so, you ensure that your life-saving technologies reach the patients who need them most without unnecessary regulatory hurdles.
Frequently Asked Questions (FAQs)
1. What is the primary focus of a QMSR inspection? The primary focus is on how well your Quality Management System integrates risk-based thinking and aligns with the requirements of ISO 13485:2016.
2. How long do manufacturers have to transition to the new guidance? The FDA has provided a transition period, but by 2026, all manufacturers are expected to be in full compliance with the QMSR requirements.
3. Does the FDA require ISO 13485 certification? No, the FDA does not mandate ISO certification, but they will inspect your facility to ensure you meet the QMSR requirements, which are now largely identical to the standard.
4. How does the QMSR affect software validation? The QMSR reinforces the need for robust software validation and data integrity, particularly for automated systems used in the quality management process.
5. What is the most common reason for a Form 483 in the QMSR era? Failures in CAPA investigations, inadequate risk management integration, and poor complaint handling remain the leading causes of regulatory findings.
6. Can I use my old QSR checklists for a QMSR inspection? You should update your checklists to include the specific ISO 13485 clauses and risk management requirements that are now part of the QMSR framework.
References and Technical Citations
FDA QMSR Final Rule Official Guide: https://www.fda.gov/medical-devices/quality-system-regulation-amendments This official document details the legal transition from QSR to QMSR and the mandatory harmonization with ISO 13485:2016 for all manufacturers.
ISO 13485:2016 – Quality Management Systems Standard: https://www.iso.org/standard/59752.html The international standard for medical device quality management that serves as the foundation for the new FDA QMSR framework.
FDA Inspection Guides (QSIT) Technical Manual: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/quality-system-inspection-technique-qsit A guide detailing how federal investigators evaluate quality system subsystems during an on-site facility audit.
FDA Data Integrity and Compliance with cGMP Guidance: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-compliance-cgmp-guidance-industry Technical guidance ensuring that all quality records—whether electronic or paper—are accurate, reliable, and secure under the new guidance.
IMDRF Principles of Software Validation: https://www.imdrf.org/documents/software-as-a-medical-device-samd-key-definitions-and-framework The international framework for validating digital systems, essential for maintaining compliance in an increasingly automated industry.
European Medical Device Regulation (EU MDR 2017/745): https://health.ec.europa.eu/medical-devices-sector/new-regulations_en The EU’s comprehensive regulation that works in tandem with QMSR to drive global quality management harmonization through ISO 13485.