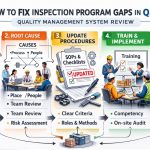
Introduction to QMSR Compliance Best Practices
The transition to the Quality Management System Regulation (QMSR) marks a significant evolution in medical device oversight. Consequently, manufacturers must now adopt specific QMSR compliance best practices to stay ahead of federal investigators. This shift harmonizes the FDA’s long-standing requirements with international ISO 13485:2016 standards. Therefore, maintaining compliance is no longer just about following domestic rules; it is about meeting a global benchmark for quality.
For organizations, this transition requires a move from reactive quality checks to proactive risk management. The FDA now evaluates how well a company integrates risk-based thinking into every operational layer. As we move deeper into 2026, the cost of non-compliance is rising. Firms must ensure their internal processes are resilient and data-driven to avoid costly 483 observations. Successful market access now depends on how effectively you can prove your system is in a state of continuous control.
In this comprehensive guide, we detail the strategies for a robust inspection program. We evaluate how to leverage technology while maintaining strict regulatory boundaries. Whether you are building a new system or updating an old one, these practices provide a roadmap for long-term success. By following these guidelines, you can ensure your Innovations reach patients safely and efficiently.
Strategic Alignment with FDA QMSR Requirements
Successful compliance starts with a deep understanding of the regulatory framework. The FDA designed the QMSR to reduce the gap between international and domestic standards. Specifically, the FDA QMSR Inspection Program Rule Interpretation emphasizes the effectiveness of the quality management system (QMS) over mere documentation. Therefore, your first best practice is to align your internal language with ISO 13485:2016 terminology.
Organizations must map their existing 21 CFR 820 procedures to the new QMSR structure. This gap analysis prevents systemic failures during unannounced audits. Furthermore, management must play an active role in this alignment. The FDA expects senior leadership to provide adequate resources and demonstrate a clear commitment to quality culture. When management is involved, the entire organization becomes more audit-ready.
To facilitate this alignment, many firms utilize a Medical Device QMSR Inspection Program Template. This tool ensures that every subsystem is evaluated according to current federal expectations. It helps identify vulnerabilities in areas like design transfer and management review before they become regulatory liabilities.
Implementing a Risk-Based Auditing Strategy
Risk management is the “red thread” that runs through the entire QMSR framework. Consequently, your internal audit program must be risk-based. You should prioritize auditing high-risk processes that directly impact patient safety and device performance. This targeted approach is much more effective than a generic checklist.
Investigators now look for evidence that risk assessments inform your CAPA (Corrective and Preventive Action) decisions. If a process shows high variability, it should trigger an immediate investigation. Furthermore, your auditors must be trained to trace a risk from the design phase all the way to the production floor. This end-to-end traceability is a hallmark of a mature quality system.
If you are unsure how to start this process, learning how to implement medical device inspection program QMSR can provide the foundational steps. A well-structured implementation plan ensures that risk management is not a standalone activity but an integrated part of your daily manufacturing cycle.
Maximizing Readiness with Mock FDA Audits
One of the most effective QMSR compliance best practices is the use of mock audits. A mock audit serves as a high-pressure rehearsal for the real thing. It tests not only your documentation but also your staff’s ability to interact with investigators. Mock audits should mimic the FDA’s “front room/back room” logistics to be truly effective.
During these simulations, you should evaluate your document retrieval speed and subject matter expert (SME) performance. If it takes too long to find a validation report, it signals a lack of control to the auditor. Therefore, practicing these logistics is just as important as having the right data. Many firms find that external eyes are needed to provide an objective critique.
Before scheduling your next rehearsal, explore Do You Need a Mock FDA Audit? Benefits and What to Expect. A mock audit identifies systemic gaps that internal teams often miss due to familiarity bias. It provides the confidence needed to handle federal investigators with professionalism and accuracy.
Operational Steps for Continuous Audit Readiness
Maintaining a state of “continuous readiness” is a vital operational goal. Consequently, you should follow a Step-by-Step Medical Device Inspection Program for QMSR Compliance throughout the year. This prevents the “panic-prep” that often occurs when an inspection is rumored or announced.
Key operational steps include:
- Contemporaneous Documentation: Ensure all records are signed and dated at the time of the activity.
- Personnel Training: Verify that training records are up-to-date and employees can demonstrate their competence.
- Supplier Monitoring: Conduct risk-based evaluations of your third-party vendors regularly.
Consistency is the enemy of the Form 483. When your processes are predictable and well-documented, the inspection becomes a verification of your success rather than an investigation of your failures. By making quality a daily habit, you protect both the patient and your organization’s reputation.
Digital Integrity and Automation Compliance
In 2026, the use of automated systems in quality management is the standard. However, automation introduces unique regulatory challenges. Your QMSR compliance best practices must include strict adherence to 21 CFR Part 11 for electronic records and signatures. Investigators frequently audit digital audit trails to ensure data has not been altered.
You must validate all software used in your QMS for its intended use. This validation must be documented and updated whenever system changes occur. Furthermore, data integrity (ALCOA+) must be maintained at all times. Records must be Attributable, Legible, Contemporaneous, Original, and Accurate. If your digital system fails these tests, your entire quality record becomes suspect.
Utilizing a QMSR Inspection Program Checklist for Medical Devices specifically for your digital tools can mitigate these risks. This checklist should cover access controls, data backup procedures, and system security. Technology should support your compliance efforts, not create new vulnerabilities for investigators to find.
Conclusion: The Future of QMSR Excellence
Mastering QMSR compliance best practices is essential for any medical device manufacturer aiming for global success in 2026. The harmonization of FDA and ISO standards provides a clearer path to quality, but it also demands higher precision. By focusing on risk-based auditing, management commitment, and digital integrity, you can build a QMS that stands up to the toughest scrutiny.
Compliance is not a finish line; it is a continuous journey of improvement. The firms that thrive are those that view regulatory requirements as a baseline for excellence rather than a burden. Stay proactive, invest in your people and technology, and maintain a culture where quality is everyone’s responsibility. By doing so, you ensure that your life-saving technologies reach those who need them most without unnecessary regulatory delays.
Frequently Asked Questions (FAQs)
1. What is the most important change in the QMSR? The most critical change is the explicit incorporation of ISO 13485:2016 by reference, which forces a shift toward a risk-based quality management approach.
2. How often should we conduct internal QMSR audits? While the regulation doesn’t specify a frequency, industry best practice is to audit every major subsystem at least once per year.
3. Does the FDA require ISO 13485 certification? No, the FDA does not mandate certification, but they will inspect your facility to ensure you meet the QMSR requirements, which are now largely identical to the ISO standard.
4. How does risk management affect CAPA? In the QMSR framework, the depth of a CAPA investigation and the urgency of the corrective action should be directly proportionate to the risk identified.
5. Can we use electronic signatures for all QMS documents? Yes, provided your system is fully validated and complies with the requirements of 21 CFR Part 11 regarding data integrity and security.
6. What is the role of management in a QMSR audit? Management must prove they provide adequate resources for the QMS and that they regularly review the system’s effectiveness through data-driven management reviews.
References and Technical Citations
FDA Quality Management System Regulation (QMSR) Final Rule (2024): https://www.federalregister.gov/documents/2024/02/02/2024-01709/medical-devices-quality-system-regulation-amendments This is the official federal document detailing the transition from QSR to QMSR and the specific legal requirements for manufacturers.
ISO 13485:2016 Medical Devices Quality Management Systems Standard: https://www.iso.org/standard/59752.html The primary international standard for medical device quality management that serves as the foundation for the new FDA QMSR framework.
FDA Inspection Guides (QSIT) Technical Manual: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/quality-system-inspection-technique-qsit A comprehensive guide detailing how federal investigators evaluate quality system subsystems during an on-site facility audit.
FDA Guidance on Data Integrity and Compliance with cGMP: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-compliance-cgmp-guidance-industry Technical guidance for manufacturers to ensure their records—both paper and electronic—meet the required standards for accuracy, reliability, and security.
IMDRF Software as a Medical Device (SaMD) Framework: https://www.imdrf.org/documents/software-as-a-medical-device-samd-key-definitions-and-framework The international framework for validating digital systems in a medical device environment, which supports QMSR goals for software validation and data integrity.
European Medical Device Regulation (EU MDR 2017/745): https://health.ec.europa.eu/medical-devices-sector/new-regulations_en The EU’s comprehensive regulation for medical devices, which works alongside the FDA’s QMSR to drive global harmonization of quality management standards.