The New Landscape of FDA Dietary Supplement Inspections
The supplement industry is currently facing unprecedented scrutiny from federal regulators. Following a series of high-profile Salmonella outbreaks and the discovery of sophisticated counterfeit operations, the agency has intensified FDA dietary supplement inspections. Consequently, manufacturers can no longer rely on legacy quality systems to pass audits. The current focus has shifted toward proactive contaminant testing and rigorous supplier verification to mitigate public health risks.
Recent alerts have highlighted a critical vulnerability in the global supply chain. Specifically, the FDA has identified several instances where raw botanical ingredients were tainted with pathogens or replaced with low-quality substitutes. Therefore, the FDA dietary supplement inspections of 2026 are specifically targeting the “Identity and Purity” sections of the cGMP regulations. Manufacturers must prove they have validated their sources and conducted comprehensive testing before any batch enters production.
This article explores the mandatory fixes your facility must implement immediately. We will discuss the technical requirements for pathogen control and the legal implications of counterfeit detection. By aligning your facility with these new expectations, you can safeguard your brand and ensure a successful outcome during your next unannounced visit.
Mandatory Fix 1: Pathogen Control and Salmonella Mitigation
Salmonella contamination remains a top priority for inspectors. Under 21 CFR Part 111, manufacturers must establish stringent controls for any ingredient susceptible to microbial growth. Recent alerts suggest that many firms fail to adequately monitor environmental conditions within their drying and mixing rooms. As a result, cross-contamination often occurs between raw materials and finished goods.
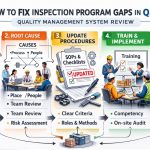
To address this, you must implement a “Zone-Based” environmental monitoring program. This involves regular swabbing of high-traffic areas and equipment surfaces to detect pathogens before they reach the product. Furthermore, if you are also managing medical device divisions, you might find that QMSR audit readiness tips for medical device inspection programs provide useful parallels in cleanroom maintenance and microbial barrier protocols.
Additionally, heat treatment and irradiation processes must be fully validated. Inspectors will ask for scientific data proving that your kill-step is effective against the specific strains of Salmonella identified in recent FDA bulletins. Do not wait for an inspection to find these gaps; proactive testing is the only way to avoid a mandatory recall.
Mandatory Fix 2: Counterfeit Detection and Supply Chain Integrity
The rise of counterfeit supplements has forced the FDA to re-evaluate the FDA dietary supplement inspections framework for purchasing controls. Fraudulent ingredients often enter the market through “gray market” distributors who offer prices significantly below market value. Consequently, the FDA now expects manufacturers to conduct physical audits of their primary suppliers.
You must implement a multi-tiered verification process. This includes:
- Chemical fingerprinting of botanical extracts to ensure species authenticity.
- Tamper-evident packaging for all raw material shipments.
- Annual on-site audits of your high-risk ingredient suppliers.
Supply chain transparency is no longer optional. If you are a newer player in the market, utilizing a dietary supplement GMP audit checklist for new manufacturers can help you establish the foundational paperwork required to prove chain of custody. Remember, a Certificate of Analysis (CoA) from a supplier is not sufficient evidence of quality; the FDA requires independent verification by the manufacturer.
Digital Integrity and Automation in GMP Compliance
As manufacturing volumes increase, manual record-keeping becomes a significant liability during FDA dietary supplement inspections. The agency is increasingly identifying “Data Integrity” issues where paper logs are either incomplete or backdated. Consequently, many firms are transitioning to automated Quality Management Systems (QMS) to ensure real-time data capture.
However, moving to a digital system requires strict adherence to 21 CFR Part 11. This includes maintaining electronic signatures and unalterable audit trails. Navigating this transition is complex; for a detailed analysis of the technical hurdles, read about automation vs. compliance: managing FDA risks in digital systems.
Automation can significantly reduce the risk of human error in labeling and batch recording. Nevertheless, the FDA will audit the validation protocols for your software. You must prove that the system accurately captures data and prevents unauthorized changes. In the current 2026 landscape, a validated digital system is a strong indicator of a high-maturity quality culture.
Identifying and Avoiding Common Audit Findings
Historical data shows that certain GMP violations appear repeatedly in warning letters. During FDA dietary supplement inspections, the most frequent citations involve sub-standard Laboratory Controls and a lack of written procedures. Specifically, firms often fail to establish scientifically valid specifications for each component.
To protect your facility, you should conduct a “Gap Analysis” against recent enforcement trends. Understanding how others have failed is an effective way to stay compliant. For instance, reviewing common FDA audit findings in medical devices and how to avoid them can reveal systemic quality issues that are also applicable to the supplement sector, such as poor root cause analysis in investigations.
If an inspector identifies a deficiency, your response must be robust. You must not only fix the specific error but also explain how you will prevent it from recurring facility-wide. This involves a comprehensive review of your training programs and standard operating procedures (SOPs).
The Strategic Value of Mock Audits in 2026
Given the recent Salmonella alerts, an unannounced FDA visit is highly likely for manufacturers of high-risk botanicals. Therefore, the most successful firms are conducting “Mock Audits” to identify vulnerabilities. A mock audit simulates the pressure of a real inspection, testing your team’s ability to retrieve documents and answer technical questions.
These simulations often reveal that staff members are unprepared for the “Front Room/Back Room” logistics required during an audit. If you are unsure whether your facility is ready for this investment, consider: do you need a mock FDA audit? benefits and what to expect.
A mock audit should specifically focus on your response to a “Pathogen Positive” result. How does your team handle the quarantine of affected batches? Is your recall plan documented and tested? Answering these questions in a simulation is far safer than answering them in front of a federal investigator.
Preparing for Specialized Inspections: PAI and Beyond
For firms launching innovative new ingredients, the FDA may conduct a Pre-Approval Inspection (PAI). This is a highly technical audit focused on the data submitted in your New Dietary Ingredient (NDI) notification. Any discrepancy between your lab notebooks and your submission can lead to a rejection of your filing.
The PAI requires a different level of preparation compared to a routine GMP audit. You must ensure that your research and development (R&D) data is as compliant as your production data. Learn how to prepare for a pre-approval FDA inspection without the panic to ensure your product launch stays on schedule.
During a PAI, inspectors will scrutinize your pilot-scale manufacturing records. They want to see that the product you intend to market is identical to the product you tested for safety. Any change in the manufacturing process must be documented and justified with scientific data.
Post-Inspection: Managing 483s and Warning Letters
If your FDA dietary supplement inspections result in a Form 483, the clock starts ticking immediately. You typically have 15 business days to provide a written response. This response must be “Action-Oriented,” meaning it must provide evidence of corrections already made, rather than just promises of future improvements.
A weak response often escalates to a Warning Letter or an Injunction. Therefore, your quality team must prioritize the CAPA (Corrective and Preventive Action) process. Every observation must be tied to a root cause. If the inspector cited Salmonella controls, your response should include updated environmental swabbing logs and revised sanitation SOPs.
Furthermore, consider the long-term impact on your brand. A public warning letter can damage retailer relationships and consumer trust. Consequently, the financial cost of a failed inspection far outweighs the cost of maintaining a robust, proactive quality system.
Conclusion: Ensuring Long-Term Compliance
The recent FDA alerts serve as a wake-up call for the supplement industry. FDA dietary supplement inspections are no longer just about checking boxes; they are about proving that you have total control over your supply chain and manufacturing environment. By fixing gaps in pathogen control, supply chain integrity, and digital data management, you can transform compliance from a burden into a competitive advantage.
In 2026, transparency is the new currency of the market. Manufacturers who can demonstrate a high level of GMP maturity will not only pass inspections but also win the trust of increasingly cautious consumers. Stay proactive, stay audited, and ensure that your facility remains a leader in supplement safety.
Frequently Asked Questions (FAQs)
1. What is the most common reason for a failed supplement inspection? The most common reasons include a lack of established specifications for raw materials and failure to conduct identity testing on every batch of ingredients.
2. How has the Salmonella alert changed FDA inspection priorities? The FDA is now specifically looking for environmental monitoring programs and validated kill-steps in facilities that handle botanical or protein powders.
3. Does the FDA require third-party certification for supplements? No, the FDA does not require third-party certification (like NSF or USP), but having these certifications can demonstrate a commitment to GMP that may facilitate a smoother inspection.
4. What should I do if I suspect a counterfeit ingredient? You should immediately quarantine the material, notify the FDA, and conduct a full traceback audit of the distributor and original manufacturer.
5. How long does an FDA dietary supplement inspection typically last? A routine inspection usually lasts between 3 to 5 days, depending on the size of the facility and the complexity of the manufacturing processes.
6. Can a digital QMS replace manual batch records? Yes, provided the software is fully validated according to 21 CFR Part 11 and includes unalterable audit trails.
References and Technical Citations
FDA Final Rule: 21 CFR Part 111 (cGMP for Dietary Supplements): https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=111 The primary regulation governing all dietary supplement manufacturing and the basis for all inspections.
FDA Salmonella Safety Alerts and Recall Database: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts Real-time information on pathogen alerts and specific strains of Salmonella currently affecting the market.
FDA Guidance: Supplier Verification and Supply Chain Integrity: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-supply-chain-program-requirements-and-supplier-verification Mandatory steps for supplier verification and documentation required to prove full chain of custody.
FDA Data Integrity and Compliance with cGMP Q&A: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-compliance-cgmp-guidance-industry Technical guidance for manufacturers transitioning from manual logs to automated digital record-keeping systems.
IMDRF Principles for Software Validation Protocols: https://www.imdrf.org/documents/software-as-a-medical-device-samd-key-definitions-and-framework The gold standard for software validation processes in any FDA-regulated manufacturing environment.
FDA Investigations Operations Manual (IOM) for Audits: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/investigations-operations-manual The primary handbook used by investigators outlining the procedures they follow during a facility audit.